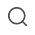Service
Objective-focused CDMO partner in the development and delivery of innovative solutions to enhance user experience
Perfect Quality System
Quality management system:
passed ISO 13485 certification, CE certification and FDA 510K certification, meeting the requirements of MDR regulations.
Reducing the risk of hospital acquired infections (HAI’s) is essential to patient safety in today’s health care environment. At DSB, we adhere to high standards and maintain rigorous controls to ensure product integrity from manufacture through to the time of procedural use. Here are some of the key standards that we adhere to:
● FDA CFR 21 Section 820 Quality System-Regulation
● ISO 13485 Medical Devices Quality System Requirements
● ISO 14644 Clean room and associated controlled environments
● ISO 11607- Packaging for Terminally Sterilized Medical Devices Parts1&2;
● ISO-11135- Sterilization of Healthcare Products- Ethylene Oxide
● ISO 10993-7- Biological evaluation of medical devices
Whether your need is an individual product or a series of components packaged as a kit, DSB can work with you to achieve the delicate balance of cost and compliance.

Goal
DSB Medical is committed to manufacturing medical products that meet customer requirements and the legal and regulatory requirements of the customer's target market. The cornerstone of our quality management system is to build quality into our products, processes and commitment to continuous improvement.
1. Customer-centric
2. To achieve continuous product improvement
3. Quality takes precedence over profit
4. Company 5s management
5. Quality Six Sigma Management
Customized Development&CDMO
Thanks to our long-term cooperation with customers in many different projects, we can flexibly design customized services
Performance:
● Customizing test methods
● Cooperation on R&D projects for testing (method implementation report)
● Regulatory affairs (such as declaration support)
● Quality: quality assurance and quality control (development of test methods for incoming and quality assurance release; mutual calibration - comparability study)
● R&D (development of test methods for new products)
● Training (specifications, regulations, test procedures, etc.)

Technologies & Manufacturing Equipment
Mold Development
Has several high-precision CNC machine tools imported from Japan, focusing on the development of multi-cavity medical equipment molds
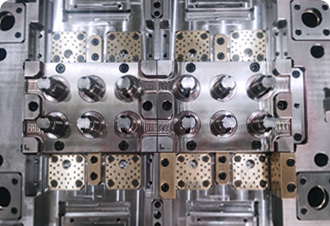
Discover MoreAutomation Development
A number of fully automated production lines with independent research and development and intellectual property rights
Discover MoreEquipment/ Processing Capability
Has the industry's high-end special film, precision plastic parts production equipment and production process
Discover MorePackaging Capablility
100,000-level purification workshop intelligent packaging production line with customized solutions
Discover Morelnspection and Analytical Capability

Liquid chromatography

Gas Chromatograph
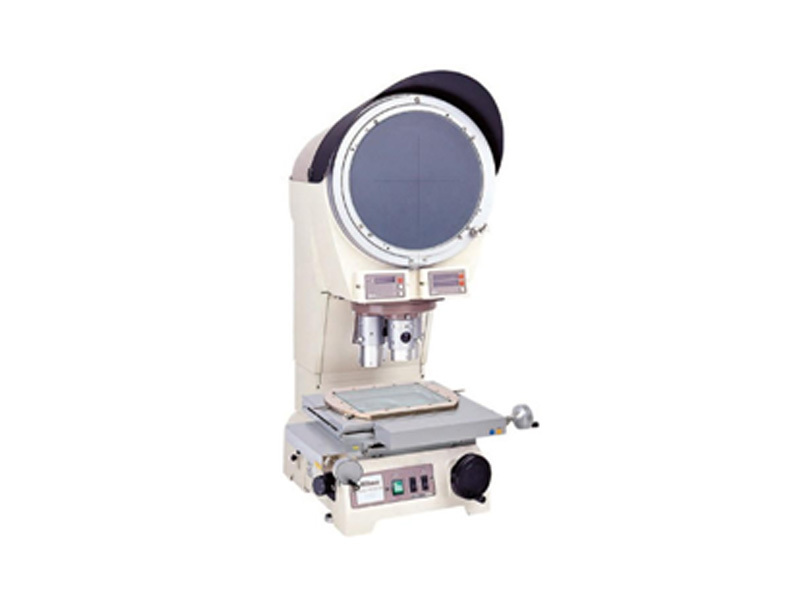
Nikon projector

Biological safety cabinet

Biochemical incubator

Tightness Tester

Synthetic Blood Penetration Tester for Masks

Pretreatment test chamber (aging test chamber)

Dust particle counter

Comprehensive Performance Tester for Luer Joints

Sprint iQ

Three- coordinate measuring system

ACI impactor

Gas circulation tester

VMS image measuring instrument
Applying for precision injection molding requirements, testing of precision multi -cavity plastic parts

Tensile test machine

LB-3308 Bacteria filtration efficiency tester

LB-3307 Particle Filtration Efficiency Tester