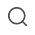-
Products & Services

-
-
News & Events

-
Face mask
Product Details
The Face Masks are intended to be worn to protect both the patient and healthcare personnel from transfer of microorganisms, body fluids and particulate material. These face masks are intended for use in infection control practices to reduce the potential exposure to blood and body fluids.
MEDICAL FACE MASK TESTS AND REQUIREMENTS
U.S.A.:ASTM F2100-19 STANDARD SPECIFICATION FOR PERFORMANCE OF MATERIALS USED IN MEDICAL FACE MASKS
EUROPE:EN 14683:2019 MEDICAL FACE MASKS-REQUIREMENTS AND TEST METHODS
| ASTM F2100-19 | EN 14683:2019 Barrier Levels | ||||||
| Level 1 | Level 2 | Level 3 | Typel | Type ll | Type IIR | ||
| Barrier Testing | BFE% ASTM F2101,EN 14683 | ≥95 | ≥98 | ≥95 | ≥98 | ||
| PFE% ASTM F2299 | ≥95 | ≥98 | Not required | ||||
| Synthetic Blood ASTM F1862,IS022609 | Pass at 80 mmHg | Pass at 120 mmHg | Pass at 160 mmHg | Not required | Pass at ≥16.0 kPa (>120 mmHg) | ||
| Physical Testing | Differential Pressure EN 14683 | <5.0mmH20/c㎡ | <6.0mmH2O/c㎡ | <40 Pa/c㎡ | <60 Pa/c㎡ | ||
| Safety Testing | Flammability 16 CFR Part 1610 | Class 1 (≥3.5 seconds) | See European Medical Directive (2007/47/EC,MDD 93/42/EEC) | ||||
| Microbial Cleanliness ISO 11737-1 | Not required | ≤30 cfu/g | |||||
| Biocompatibility ISO 10993 | 510 K Guidance recommends testing to ISO 10993 | Complete an evaluation according to ISO 10993 | |||||
| Sampling ANSI/ASQC Z1.4 ISO 2859-1 | AQL 4% for BFE,PFE,Delta P 32 masks for Synthetic Blood (Pass= ≥29 passing, Fail = ≤28 passing) 14 masks for Flammability | Minimum of 5 masks up to an AQL of 4% for BFE, Delta P and Microbial Cleanliness 32 masks for Synthetic Blood (Pass = ≥29 passing,Fail=≤28 passing) | |||||
Recommended Products
If you have more needs?
Welcome your message, we will reply you in time.
COOKIES
Our website uses cookies and similar technologies to personalize the advertising shown to you and to help you get the best experience on our website. For more information, see our Privacy & Cookie Policy
COOKIES
Our website uses cookies and similar technologies to personalize the advertising shown to you and to help you get the best experience on our website. For more information, see our Privacy & Cookie Policy
These cookies are necessary for basic functions such as payment. Standard cookies cannot be turned off and do not store any of your information.
These cookies collect information, such as how many people are using our site or which pages are popular, to help us improve the customer experience. Turning these cookies off will mean we can't collect information to improve your experience.
These cookies enable the website to provide enhanced functionality and personalization. They may be set by us or by third-party providers whose services we have added to our pages. If you do not allow these cookies, some or all of these services may not function properly.
These cookies help us understand what you are interested in so that we can show you relevant advertising on other websites. Turning these cookies off will mean we are unable to show you any personalized advertising.

355 Longjin Road, Changzhou Economic Development Zone, Jiangsu Province, China